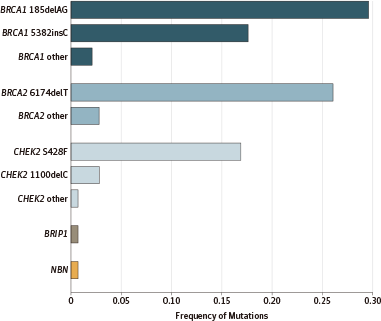除了乳腺癌易感基因,还有什么基因突变可以引起乳腺癌遗传倾向?
遗传性乳腺癌大约占所有乳腺癌的5%~10%,既往研究显示乳腺癌易感基因(BRCA)突变能解释很大比例的遗传性乳腺癌,另外还有很多已知基因与遗传性乳腺癌有关,但是仍有至少超过50%的遗传性乳腺癌尚无法解释其致病原因。那么,在未携带BRCA始祖突变的乳腺癌患者中,携带其他突变的可能性有多大?
-
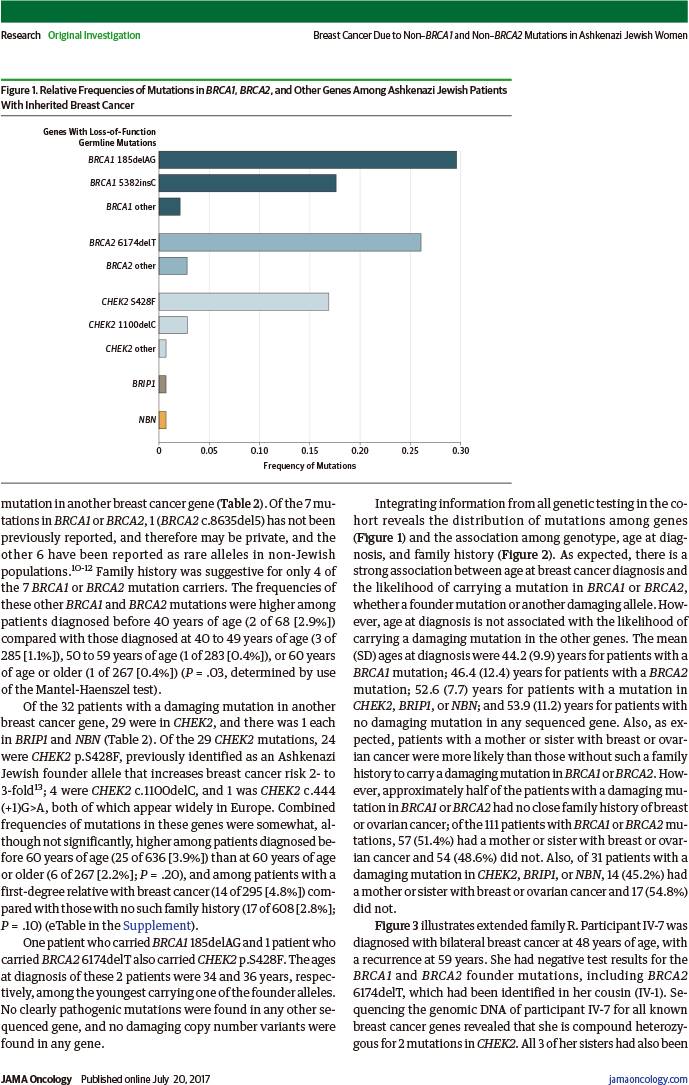
始祖突变即发生在特定人群中的突变,是指在一个人群的祖先中,有一个或多个个体携带特定突变,这些突变能够通过遗传传给后代,这种始祖突变往往会在特定人群形成突变热点,当这个人群扩大时,此突变得以在人群中播散开来。对特定的人群而言,始祖突变使遗传异质性减少,并使对突变携带者的检测和遗传咨询更简便易行。BRCA突变在某些人群具有独特的始祖突变。例如,在阿什肯纳兹犹太人群中,BRCA1的185delAG、5382insC突变和BRCA2的6174delT突变很常见。
-
阿什肯纳兹犹太人源于中世纪德国莱茵兰一带的犹太人后裔(阿什肯纳兹在近代指德国)。其中很多人自10世纪至19世纪期间,向东欧迁移。在11世纪,阿什肯纳兹犹太人仅占全世界犹太人的3%,然而到1931年达92%,现在占世界犹太人的80%。在欧洲有久远历史的犹太群体,除了地中海一带,大多数属于阿什肯纳兹。最近两个世纪来从欧洲外迁,特别是移民美国的的犹太人相当一部分来自东欧的阿什肯纳兹犹太人。在阿什肯纳兹犹太人女性中,BRCA的3个始祖突变严重增加了乳腺癌和卵巢癌的风险。然而,在未携带这些始祖突变的阿什肯纳兹犹太人乳腺癌患者中,携带其他BRCA突变或其他乳腺癌基因的可能性仍然未知。这些信息对于患者和家属的癌症防治将很有价值。
2017年7月20日,《美国医学会杂志肿瘤学分册》在线发表西雅图华盛顿大学的研究报告,发现在无BRCA始祖突变的患者中,0.8%携带其他BRCA致病突变,3.4%携带其他乳腺癌基因突变,故该人群乳腺癌患者可从所有乳腺癌基因遗传检测获益。
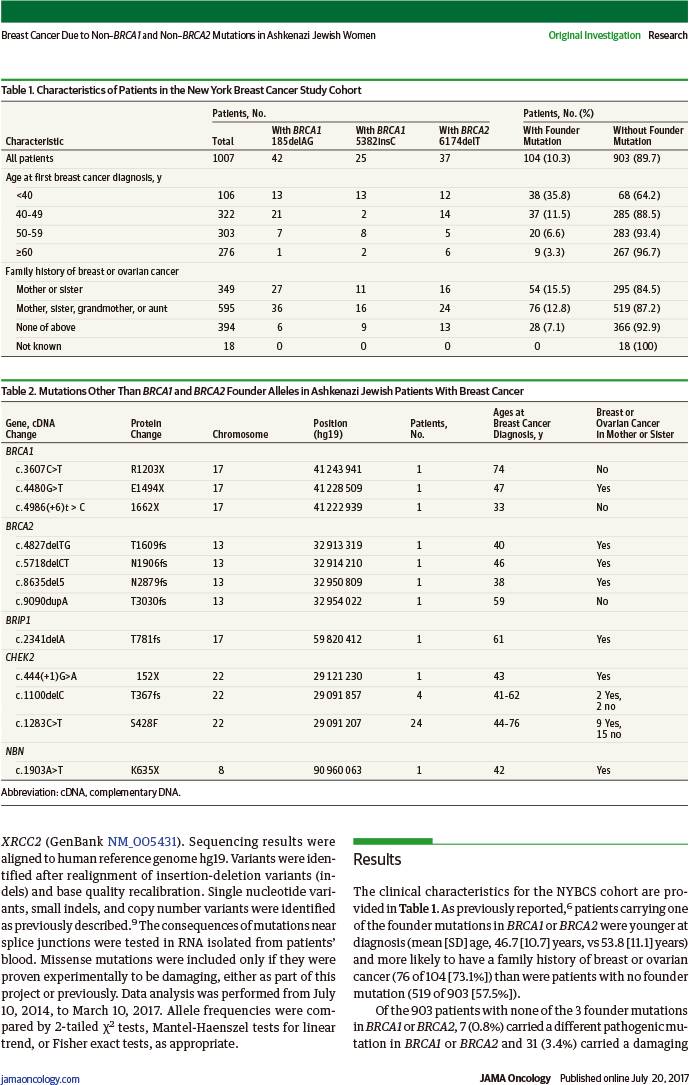
该队列研究利用1996~2000年纽约乳腺癌研究(NYBCS)12个主要癌症中心的基因组DNA,对1007例阿什肯纳兹犹太人(祖父母、外祖父母均为阿什肯纳兹犹太人)首次诊断为浸润性乳腺癌患者的23个已知和候选乳腺癌基因进行多基因组测序,于2014年7月10日~2017年3月10日进行数据分析。
结果发现:
-
在903例无BRCA始祖突变的患者中,7例(0.8%)携带其他BRCA致病突变、31例(3.4%)携带其他乳腺癌基因致病突变(29例CHEK2、1例BRIP1、1例NBN)。
-
在142例乳腺癌基因突变的患者中,104例(73.8%)携带BRCA始祖突变、7例(4.9%)携带其他BRCA致病突变、31例(21.8%)携带其他乳腺癌基因致病突变。
-
在1007例阿什肯纳兹犹太人乳腺癌患者中,142例(14.1%)携带乳腺癌基因突变:111例(11.0%)携带BRCA突变,31例(3.1%)携带CHEK2等其他乳腺癌基因突变。
-
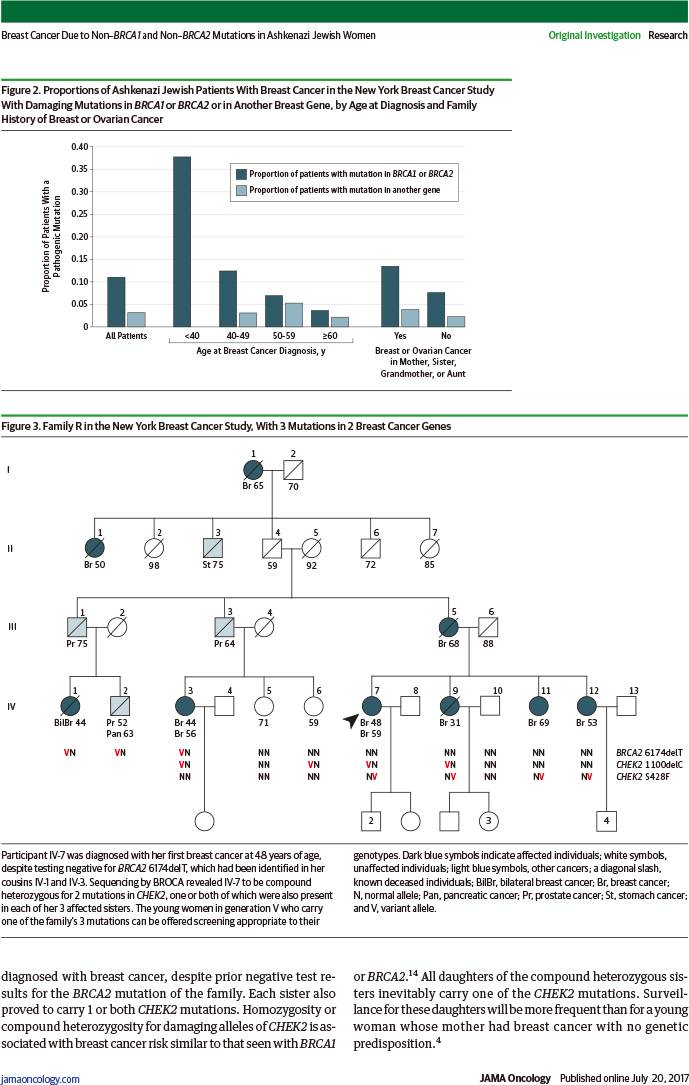
在111例携带BRCA突变患者中:57例(51.4%)母亲或姐妹有乳腺或卵巢癌,54例(48.6%)无。
因此,综合测序将为特定人群乳腺癌患者提供完整的相关遗传信息。
Breast Cancer Due to Non-BRCA1 and Non-BRCA2 Mutations in Ashkenazi Jewish Women
编者按:在对中国人群的早期研究中,往往样本量偏小,所以能够发现重复突变位点的概率很低。对中国乳腺癌或卵巢癌患者BRCA1基因突变的研究发现,香港和台湾人群均有重复的突变位点,分别是589delCT和IVS7-24del10,但是当时并未被其他研究所证实。随着研究的进一步深入、样本量的扩大以及突变携带者检出数量的增多,中国人群重复突变的位点越来越多被发现。2007年,复旦大学附属肿瘤医院完成了489例家族性或早发性乳腺癌的BRCA1/2检测,共发现23例BRCA1突变和21例BRCA2突变,其中在BRCA1基因上发现两个重复突变位点1100delAT和5589del8,各占4例。为验证这两个突变是否是中国人群的始祖突变,复旦大学附属肿瘤医院在426例散发性乳腺癌和564例健康对照中进行这两个位点的检测,结果在426例散发性乳腺癌中发现2例5589del8突变,在564例健康对照中发现1例1100delAT突变。在6例携带5589del8突变者中,有1例来自辽宁,3例来自上海,2例来自浙江;在5例1100delAT突变者中,辽宁1例、山东2例、上海1例、广东1例。这些重复突变并没有明显的地域聚集性。单倍型分析同样显示,重复位点具有相同或相似的单倍型。通过对已发表文献和美国乳腺癌信息数据库的回顾发现,1100delAT主要分布于白色人种中,唯一的亚洲报道为马来西亚的华人;5589del8则集中分布于亚洲黄色人种,其分布于北京、上海以及旅美亚裔人群均有文献报道。更有趣的是,在韩国乳腺癌患者中也发现了这个位点的突变。因此,1100delAT和5589del8,特别是5589del8,很有可能是中国人群特有的始祖突变。
相关阅读
JAMA Oncol. 2017 Jul 20. [Epub ahead of print]
Genetic Predisposition to Breast Cancer Due to Mutations Other Than BRCA1 and BRCA2 Founder Alleles Among Ashkenazi Jewish Women.
Tom Walsh; Jessica B. Mandell; Barbara M. Norquist; Silvia Casadei; Suleyman Gulsuner; Ming K. Lee; Mary-Claire King.
University of Washington, Seattle.
This cohort study uses genomic DNA from the New York Breast Cancer Study to determine the frequency of cancer-predisposing mutations other than the BRCA1 and BRCA2 founder alleles among patients of Ashkenazi Jewish ancestry with breast cancer.
QUESTION: Among patients of Ashkenazi Jewish ancestry with breast cancer who do not carry one of the founder mutations in BRCA1 or BRCA2, what is the likelihood of carrying another cancer-predisposing mutation in BRCA1, BRCA2, or another breast cancer gene?
FINDINGS: In this cohort study, 1007 patients of Ashkenazi Jewish ancestry with breast cancer were evaluated by multiplex genomic sequencing for all known and candidate breast cancer genes. Among patients without a founder mutation in BRCA1 or BRCA2, 0.8% carried a different mutation in BRCA1 or BRCA2 and 3.4% carried a mutation in another gene.
MEANING: Ashkenazi Jewish patients with breast cancer can benefit from genetic testing for all breast cancer genes.
IMPORTANCE: Among Ashkenazi Jewish women, 3 mutations in BRCA1 and BRCA2 severely increase the risk of breast and ovarian cancer. However, among Ashkenazi Jewish patients with breast cancer who do not carry one of these founder mutations, the likelihood of carrying another pathogenic mutation in BRCA1 or BRCA2 or another breast cancer gene is not known. This information would be valuable to the patient and family for cancer prevention and treatment.
OBJECTIVE: To determine the frequency of cancer-predisposing mutations other than the BRCA1 and BRCA2 founder alleles among patients of Ashkenazi Jewish ancestry with breast cancer.
DESIGN, SETTING, AND PARTICIPANTS: In this cohort study, genomic DNA of women from 12 major cancer centers with a first diagnosis of invasive breast cancer who identified themselves and all 4 grandparents as Ashkenazi Jewish and participated in the New York Breast Cancer Study (NYBCS) from 1996 to 2000 was sequenced for known and candidate breast cancer genes. Data analysis was performed from July 10, 2014, to March 10, 2017.
MAIN OUTCOMES AND MEASURES: Genomic DNA from all 1007 NYBCS probands was sequenced for 23 known and candidate breast cancer genes using BROCA, a targeted multiplexed gene panel.
RESULTS: Of the 1007 probands in the study, 903 probands had no founder mutations in BRCA1 or BRCA2; of these probands, 7 (0.8%) carried another pathogenic mutation in BRCA1 or BRCA2, and 31 (3.4%) carried a pathogenic mutation in another breast cancer gene (29 in CHEK2, and 1 each in BRIP1 and NBN). Of all inherited predispositions to breast cancer in the NYBCS, 73.8% (104 of 142) were due to a BRCA1 or BRCA2 founder allele, 4.9% (7 of 142) to another BRCA1 or BRCA2 mutation, and 21.8% (31 of 142) to a mutation in another gene. Overall, 14.1% (142 of 1007) of Ashkenazi Jewish patients with breast cancer in the NYBCS carried a germline mutation responsible for their disease: 11.0% (111 of 1007) in BRCA1 or BRCA2, and 3.1% (31 of 1007) in CHEK2 or another breast cancer gene. Of the 111 patients with BRCA1 or BRCA2 mutations, 57 (51.4%) had a mother or sister with breast or ovarian cancer and 54 patients (48.6%) did not.
CONCLUSIONS AND RELEVANCE: Comprehensive sequencing would provide complete relevant genetic information for Ashkenazi Jewish patients with breast cancer.
DOI: 10.1001/jamaoncol.2017.1996