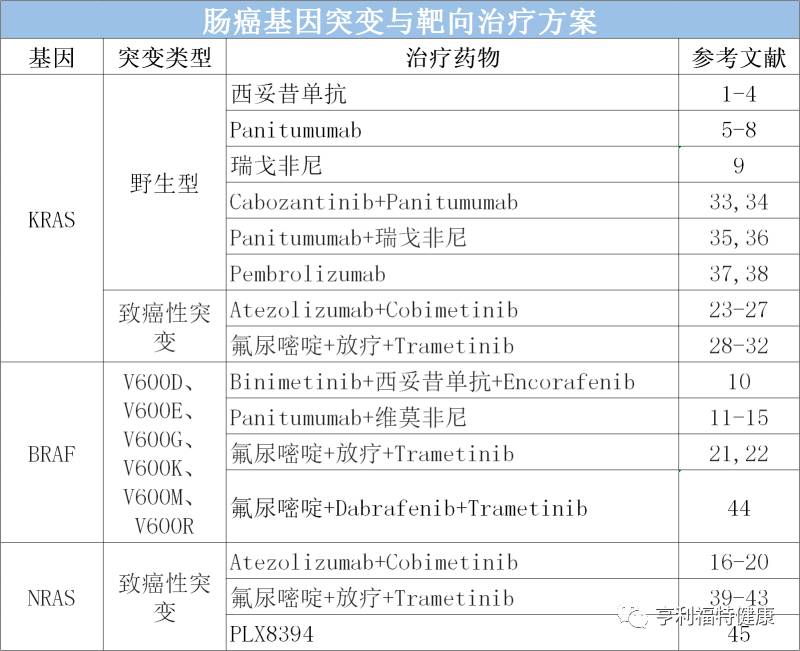
肠癌基因突变与靶向治疗方案
疾病治疗需要“同病异治”。个性化治疗可以提高治疗效果、减少不良反应、减轻经济负担。
| 1 | Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. Cunningham D et al. N Engl J Med. 2004 PMID: 15269313 |
| 2 | Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. Chung KY et al. J Clin Oncol. 2005 PMID: 15677699 |
| 3 | Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. Van Cutsem E et al. N Engl J Med. 2009 PMID: 19339720 |
| 4 | Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Price TJ et al. Lancet Oncol. 2014 PMID: 24739896 |
| 5 | Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. Van Cutsem E et al. J Clin Oncol. 2007 PMID: 17470858 |
| 6 | Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. Peeters M et al. J Clin Oncol. 2010 PMID: 20921462 |
| 7 | Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. Douillard JY et al. J Clin Oncol. 2010 PMID: 20921465 |
| 8 | Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Price TJ et al. Lancet Oncol. 2014 PMID: 24739896 |
| 9 | Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Grothey A et al. Lancet. 2013 PMID: 23177514 |
| 10 | B-Raf and the inhibitors: from bench to bedside. Huang T et al. J Hematol Oncol. 2013 PMID: 23617957 |
| 11 | Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. Di Nicolantonio F et al. J Clin Oncol. 2008 PMID: 19001320 |
| 12 | Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. Laurent-Puig P et al. J Clin Oncol. 2009 PMID: 19884556 |
| 13 | Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. De Roock W et al. Lancet Oncol. 2010 PMID: 20619739 |
| 14 | EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Corcoran RB et al. Cancer Discov. 2012 PMID: 22448344 |
| 15 | Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Yaeger R et al. Clin Cancer Res. 2015 PMID: 25589621 |
| 16 | PD-L1 Expression and Tumor-Infiltrating Lymphocytes Define Different Subsets of MAPK Inhibitor-Treated Melanoma Patients. Kakavand H et al. Clin Cancer Res. 2015 PMID: 25609064 |
| 17 | Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Hu-Lieskovan S et al. Sci Transl Med. 2015 PMID: 25787767 |
| 18 | PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. Le DT et al. N Engl J Med. 2015 PMID: 26028255 |
| 19 | RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation Between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Loi S et al. Clin Cancer Res. 2016 PMID: 26515496 |
| 20 | Bendell et al. Abstract# 3502, ASCO 2016 |
| 21 | Improved survival with MEK inhibition in BRAF-mutated melanoma. Flaherty KT et al. N Engl J Med. 2012 PMID: 22663011 |
| 22 | Novel MEK inhibitor trametinib and other retinoblastoma gene (RB)-reactivating agents enhance efficacy of 5-fluorouracil on human colon cancer cells. Watanabe M et al. Cancer Sci. 2013 PMID: 23438367 |
| 23 | PD-L1 Expression and Tumor-Infiltrating Lymphocytes Define Different Subsets of MAPK Inhibitor-Treated Melanoma Patients. Kakavand H et al. Clin Cancer Res. 2015 PMID: 25609064 |
| 24 | Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Hu-Lieskovan S et al. Sci Transl Med. 2015 PMID: 25787767 |
| 25 |
PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. Le DT et al. N Engl J Med. 2015 PMID: 26028255 |
| 26 | RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation Between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Loi S et al. Clin Cancer Res. 2016 PMID: 26515496 |
| 27 | Bendell et al. Abstract# 3502, ASCO 2016 |
| 28 | Enhancement of 5-fluorouracil-induced in vitro and in vivo radiosensitization with MEK inhibition. Urick ME et al. Clin Cancer Res. 2011 PMID: 21690569 |
| 29 | Improved survival with MEK inhibition in BRAF-mutated melanoma. Flaherty KT et al. N Engl J Med. 2012 PMID: 22663011 |
| 30 | Novel MEK inhibitor trametinib and other retinoblastoma gene (RB)-reactivating agents enhance efficacy of 5-fluorouracil on human colon cancer cells. Watanabe M et al. Cancer Sci. 2013 PMID: 23438367 |
| 31 | Trametinib: first global approval. Wright CJ et al. Drugs. 2013 PMID: 23846731 |
| 32 | Germann et al. Abstract# 4693, AACR 2015 |
| 33 | Potent antitumor activity of cabozantinib, a c-MET and VEGFR2 inhibitor, in a colorectal cancer patient-derived tumor explant model. Song EK et al. Int J Cancer. 2015 PMID: 25242168 |
| 34 | Strickler et al. Abstract# 3548, ASCO 2016 |
| 35 | Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Wilhelm SM et al. Int J Cancer. 2011 PMID: 21170960 |
| 36 | Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Grothey A et al. Lancet. 2013 PMID: 23177514 |
| 37 |
PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. Le DT et al. N Engl J Med. 2015 PMID: 26028255 |
| 38 | Pembrolizumab. Khoja L et al. J Immunother Cancer. 2014 PMID: 26288737 |
| 39 | Enhancement of 5-fluorouracil-induced in vitro and in vivo radiosensitization with MEK inhibition. Urick ME et al. Clin Cancer Res. 2011 PMID: 21690569 |
| 40 | Improved survival with MEK inhibition in BRAF-mutated melanoma. Flaherty KT et al. N Engl J Med. 2012 PMID: 22663011 |
| 41 | Novel MEK inhibitor trametinib and other retinoblastoma gene (RB)-reactivating agents enhance efficacy of 5-fluorouracil on human colon cancer cells. Watanabe M et al. Cancer Sci. 2013 PMID: 23438367 |
| 42 | Trametinib: first global approval. Wright CJ et al. Drugs. 2013 PMID: 23846731 |
| 43 |
Germann et al. Abstract# 4693, AACR 2015 |
| 44 | A metastatic colon adenocarcinoma harboring BRAF V600E has a durable major response to dabrafenib/trametinib and chemotherapy. Williams CB et al. Onco Targets Ther. 2015 Dec 1;8:3561-4. doi: 10.2147/OTT.S90766. PMID: 26664139 |
| 45 | PLX8394, a new generation BRAF inhibitor, selectively inhibits BRAF in colonic adenocarcinoma cells and prevents paradoxical MAPK pathway activation. Tutuka CSA et al., Mol Cancer. 2017 Jun 28;16(1):112. doi: 10.1186/s12943-017-0684-x. PMID: 28659148 |